

The pi becomes strong if the p orbitals have complete sideways overlapping and are parallel to each other. The pi gets weak due to the partially lateral orbit formation. If electrons heavily influence the axis bond on the p orbitals, it forms a pi bond. What are Pi Bond Formations?Īs per the pi bond definition, two laterally or bonding sideways bonds are called lateral p orbitals, and the formation is called the pi orbital or covalent pi bond. D orbitals can also be placed in this bonding. The pi bond definition says that pi is related to the p orbitals as it has a parallel arrangement of atoms. The pi bond definition is a naturally covalent chemical bond that involves the overlapping lateral structure of orbitals belonging to different atoms. Unsaturated molecules hold the pi bond mostly.It is less reactive to bond formations.The pi bond decides the length of the molecule formations.
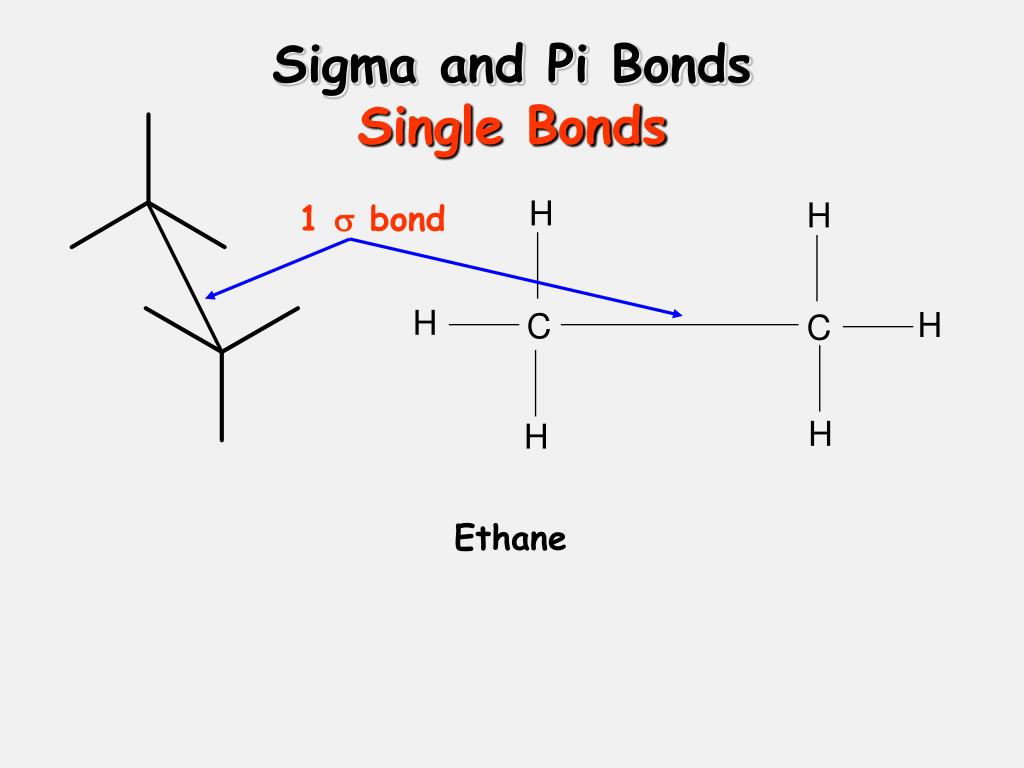
It is not the prime bond in the information that is formed later.A pi bond is not formed by saturated molecules as unsaturated molecules like alkanes and alkenes mainly include it.A pi bond is formed from the structure of the sigma bond.Due to its parallel structure, it is brittle and fragile.A pi bond is formed on the orbital axis due to the overlapping structure of orbital atoms.Let us see what are pi bonds, some of their characteristics, and the formation of their structure below for a detailed understanding. Its overlapping p-orbitals nature is the reason for its fragility of time present and considered the weaker atoms compared to others. According to quantum techniques, the p-orbitals are fragile due to the parallel structure. Pi bonds are weaker than others since their negative charge atoms are present farther away from positively charged atoms and nuclei of the atom. Orbitals are placed side by side where the electrons are placed heavily on the plane to form bonding atoms called pi bonds.


 0 kommentar(er)
0 kommentar(er)
